Ivoclar Vivadent Helioseal F v.2 User Manual
Ivoclar Vivadent Equipment
Description
Helioseal
®
F is a light-curing, white-shaded fissure sealant featuring fluoride
release.
Composition
The monomer matrix consists of Bis-GMA, urethane dimethacrylate, and
triethylene glycol dimethacrylate (58.6 wt%). The fillers are highly dispersed
silicon dioxide and fluorosilicate glass (40.5 wt%). Additional contents are
titanium dioxide, stabilizers and catalysts (< 1 wt%).
Indication
Helioseal F is used to seal pits, fissures, and foramina caeca.
Contraindication
–
If the patient is known to be allergic to any of the materials ingredients.
–
If a dry working field cannot be established.
Side effects
In individual cases, contact allergies may occur. According to today's standard
of knowledge, systemic side effects are not known.
Other interactions
None known to date
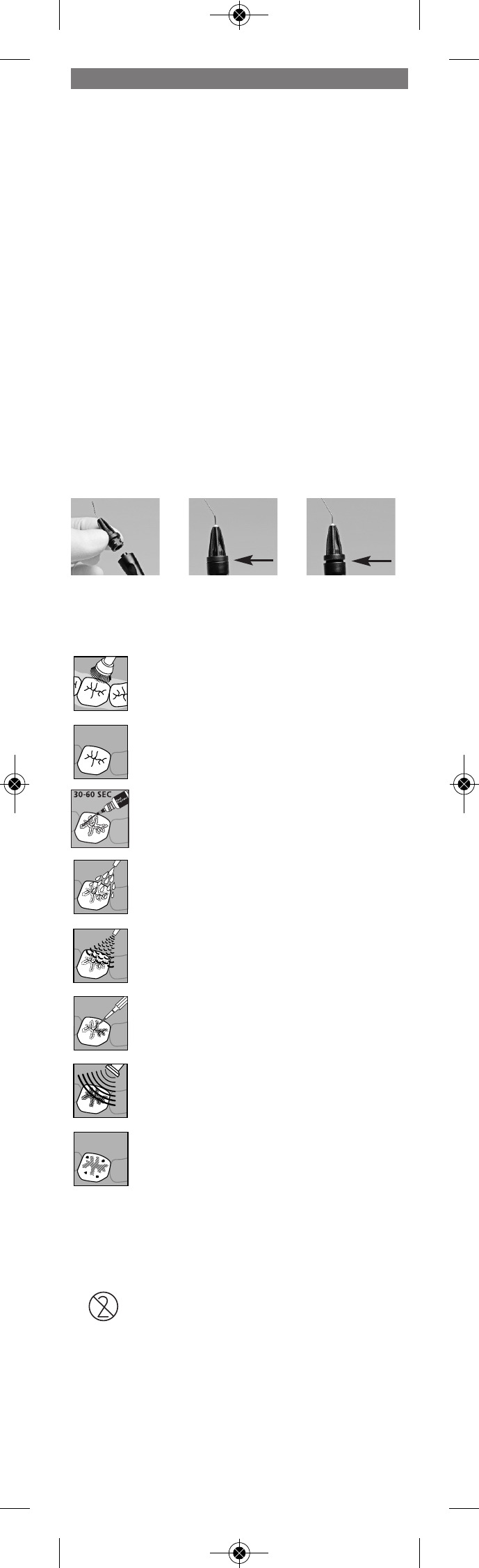
Preparatory steps
1. Remove the syringe cap by twisting it until it snaps and then pulling it off
the syringe.
2. Attach the applicator tip (Fig. 1) and twist it until it is flush with the
syringe (Fig. 2).
Step-by-step application procedure
1. Thoroughly clean the enamel surface to be sealed.
2. Isolate the working field, preferably with a rubber dam.
3. Apply an etching gel, e.g. Email Preparator, and let it react
for 30 to 60 seconds.
4. Rinse thoroughly.
5. Dry with water- and oil-free air. The etched enamel should
have a mat white appearance. Avoid contamination of the
etched surface with saliva.
6. Apply Helioseal F directly with the disposable cannula or a
disposable brush, and disperse.
7. Wait for approx. 15 seconds. Then cure the sealant with
a suitable poly merization light (e.g. bluephase
®
) for
20 seconds.
8. Check seal and occlusion.
Warning
Avoid contact of unpolymerized material with skin/mucous membrane or eyes.
Unpolymerized Helioseal F may cause slight irritation and, in rare cases, may
lead to a sensitization against methacrylates. Commercial medical gloves do
not provide protection against the sensitizing effect of methacrylates.
Special note
–
If Helioseal F is applied from the Cavifil directly in the mouth of
the patient, we recommend using this Cavifil only for one patient
due to hygienic reasons (prevention of cross-contamination be-
tween patients). The same applies to the application tips of the syringe.
–
Helioseal F syringes must be used exclusively with the application tips
provided. Other types of application tips may suddenly pop off during
dispensing.
Storage
–
Close Helioseal F syringe immediately after use
–
Store material at 2–28 °C (36–82 °F).
–
Shelf life: see date of expiration.
–
Do not use the material after the indicated date of expiration.
–
Syringes or Cavifils should not be disinfected with oxidizing disinfection
agents.
English
✗
✓
Fig. 2: right
Fig. 3: wrong
Fig. 1
Helioseal F_GI_61x245_Halb_637729_Rev0 21.03.11