Widex Ear-sets for BTE User Manual
Page 77
77
symbols commonly used by Widex a/s in medical device labelling
(labels/ifU/etc.)
symbol
title/Description
manufacturer
The product is produced by the manufacturer whose name
and address are stated next to the symbol . If appropriate,
the date of manufacture may also be stated .
Date of manufacture
The date when the product was manufactured .
Use-by date
The date after which the product is not to be used .
batch code
The product’s batch code (lot or batch identification) .
symbol
title/Description
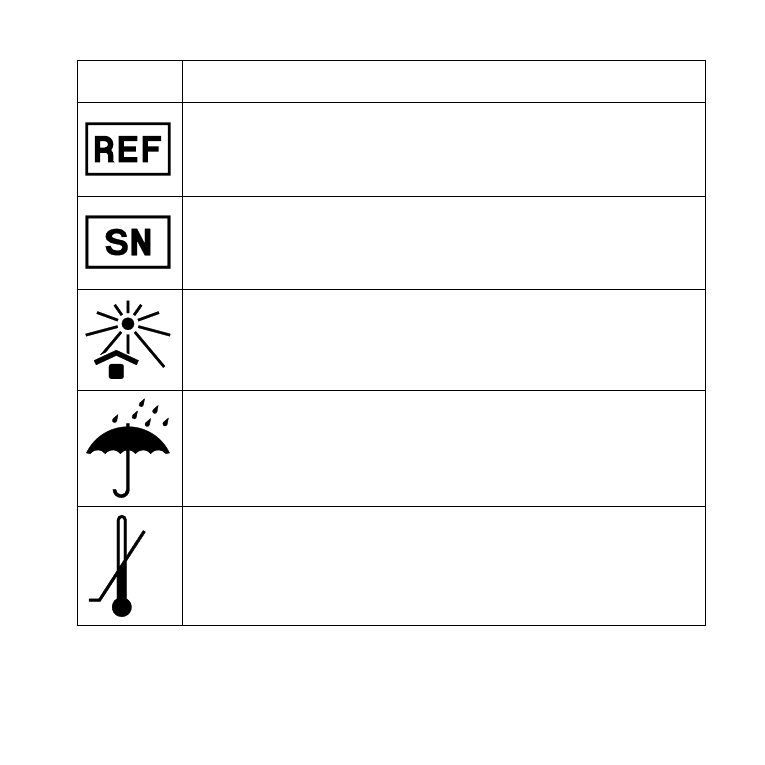
Catalogue number
The product’s catalogue (item) number .
serial number
The product’s serial number .*
Keep away from sunlight
The product must be protected from light sources and/or
The product must be kept away from heat
Keep dry
The product must be protected from moisture and/or The
product must be kept away from rain
lower limit of temperature
The lowest temperature to which the product can be safely
exposed .