Receiving inspection and storage – Fluke Biomedical 960CD-220 User Manual
Page 12
Victoreen 960CD-220,221,222,223
Operators Manual
1-8
NOTE
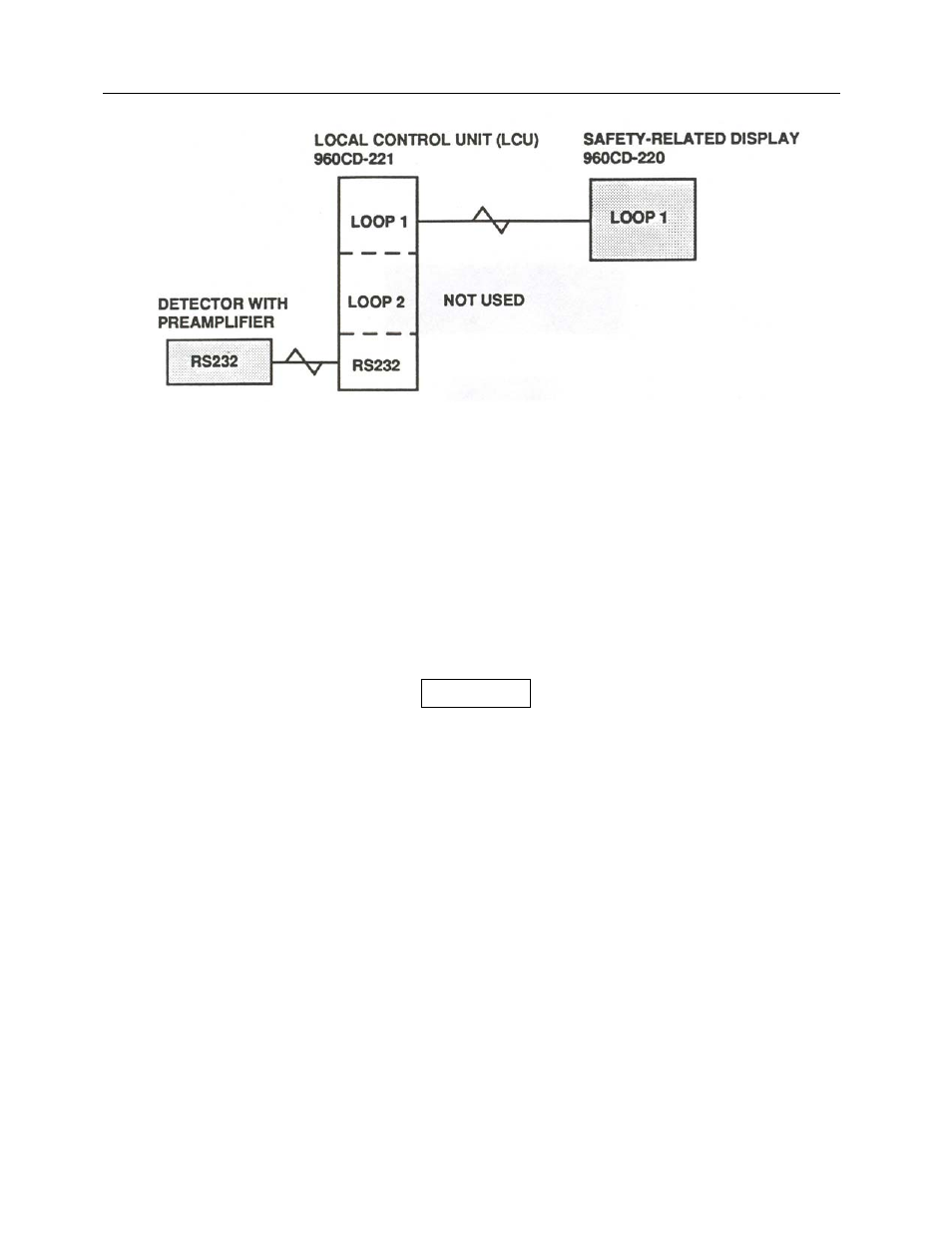
Figure 1-6. Safety Related Display Communications with RS-232 Interface to Victoreen 977 Series
Preamplifier/Electrometer
1.4
Receiving Inspection and Storage
Receiving Inspection
Upon receipt of the unit:
1. Inspect the carton(s) and contents for damage. If damage is evident, file a claim with the carrier and
notify Fluke Biomedical, Radiation Management Services at 440.248.9300.
2. Remove the contents from the packing material.
3. Verify that all items listed on the packing list have been received and are in good condition.
If any of the listed Items are missing or damaged,
notify Fluke Biomedical.
Storage
Storage of Victoreen instruments must comply with Level B storage requirements as outlined in ANSI
N45.2.2 (1972) Section 6.1.2(.2). The storage area shall comply with ANSI N45.2.2 (1972) Section 6.2
Storage Area, Paragraphs 6.2.1 through 6.2.5. Housekeeping shall conform to ANSI N45.2.3 (1972).
Level B components shall be stored within a fire resistant, tear resistant, weather tight enclosure, in a
well-ventilated building or equivalent.
Storage of Victoreen instruments must comply with the following:
1. Inspection and examination of items in storage must be in accordance with ANSI N45.2.2 (1972)
Section 6.4.1.
2. Requirements for proper storage must be documented and written procedures or instructions must
be established.
3. In the event of fire, post-fire evaluation must be in accordance with ANSI N45.2.2 (1972), Section
6.4.3.
4. Removal of items from storage must be in accordance with ANSI N45.2.2 (1972), Sections 6.5 and
6.6.