Q3 Innovations DrugHAWK ECO Cup User Manual
Page 2
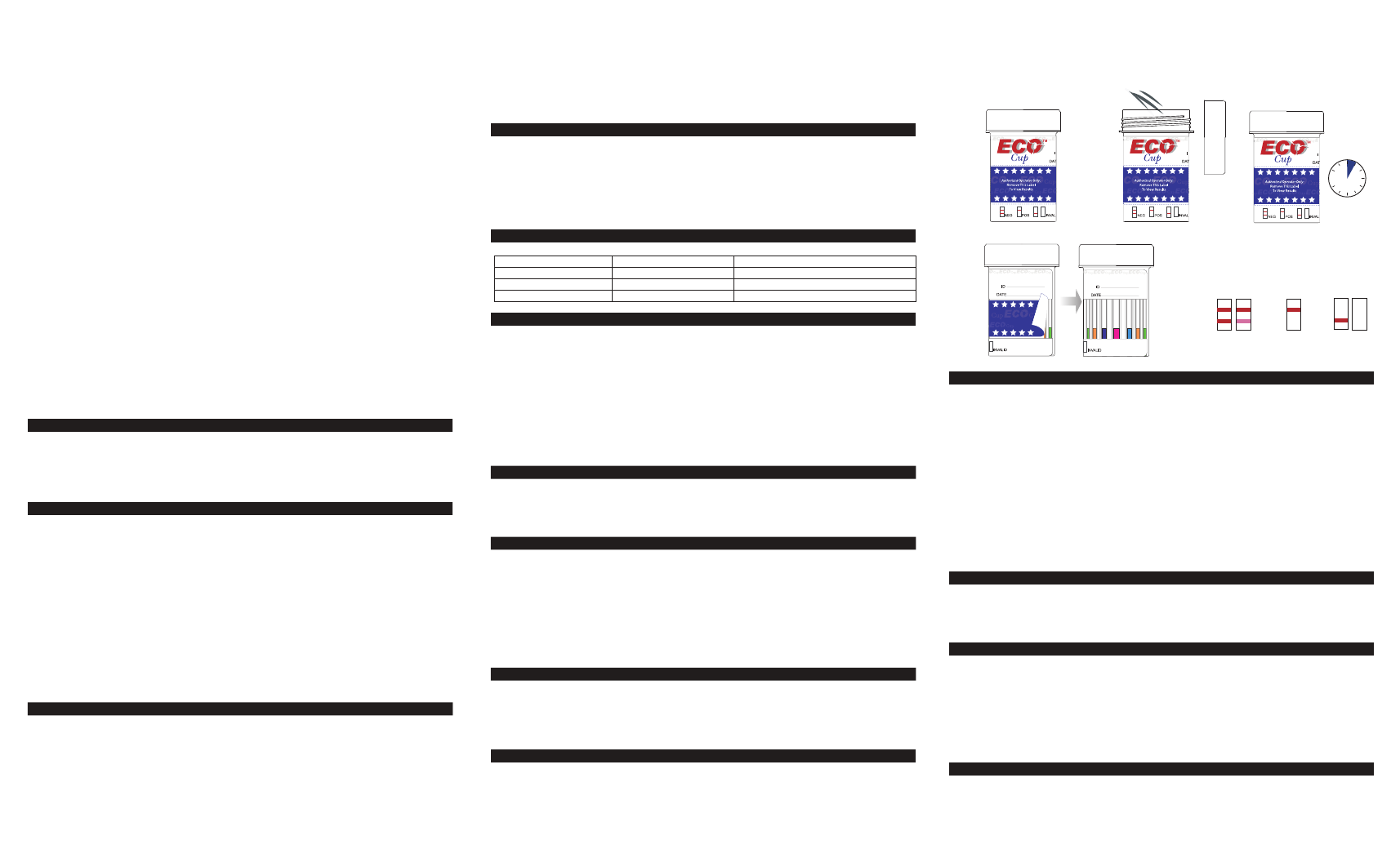
test cup. Be sure to fill up the test cup with the urine specimen between minimum 30ml to
maximum 70ml (marked on the cup).(Fig. 2)
3) After urine specimen has been collected, close the lid securely and return cup to collection
official.(Fig. 3)
4) Collection official use glove provided. Peel off label to reveal test result. Read test result at 5
minutes. DO NOT INTERPRET RESULT AFTER 10 MINUTES.(Fig. 4)
INTERPRETATION OF RESU
(Please refer to the previous illustration)
NEGATIVE: Two lines appear. * One color line should be in the control region (C), and another
apparent color line adjacent should be in the test region (T). This negative result indicates that the
drug concentration is below the detectable level.
*NOTE: The shade of color in the test line region (T) will vary, but it should be considered negative
whenever there is even a faint distinguishable color line.
POSITIVE: One color line appears in the control region (C). No line appears in the test region (T).
This positive result indicates that the drug concentration is above the detectable level.
INVALID: Control line fails to appear. Insufficient specimen volume or incorrect procedural
techniques are the most likely reasons for control line failure. Review the procedure and repeat
the test using a new test device. If the problem persists, discontinue using the lot immediately and
contact your supplier.
ADULTERANT TESTS (SPECIMEN VALIDITY TESTS) INTEPRETATION
(Please refer to the color chart)
Semi-quantitative results are obtained by visually comparing the reacted color blocks on the strip
to the printed color indicator on the color chart. No instrumentation is required.
ADULTERANT TESTS (SPECIMEN VALIDITY TESTS) LIMITATIONS
1. The adulterant tests included with the product are meant to aid in the determination of abnormal
specimens, but may not cover all the possible adulterants.
2. Oxidants: Normal human urine should not contain oxidants. The presence of high level of
antioxidants in the specimen, such as ascorbic acid, may result in false negative results for the
oxidants pad..
3. Specific Gravity: Elevated levels of protein in urine may cause abnormally high specific gravity
values.
QUALITY CONTROL
A procedural control is included in the test. A color line appearing in the control region (C) is
considered an internal procedural control. It confirms sufficient specimen volume, adequate
form in the test line region.
A drug-positive urine specimen will not generate a colored line in the specific test line region of the
strip because of drug competition, while a drug-negative urine specimen will generate a line in the
test line region because of the absence of drug competition.
To serve as a procedural control, a colored line will always appear at the control line region,
indicating that proper volume of specimen has been added and membrane wicking has occurred.
REAGENTS
The test contains a membrane strip coated with drug-protein conjugates (purified bovine albumin)
on the test line, a goat polyclonal antibody against gold-protein conjugate at the control line, and a
dye pad which contains colloidal gold particles coated with mouse monoclonal antibody specific to
Amphetamine, Cocaine, Methamphetamine, Methylenedioxymethamphetamine, Morphine, THC,
Phencyclidine, Benzodiazepines, Methadone, Barbiturates, Tricyclic Antidepressants or
Oxycodone.
ADULTERANT TESTS (SPECIMEN VALIDITY TESTS) REAGENTS
PRECAUTIONS
• For Professional Use Only.
• For In Vitro Diagnostic Use Only.
• Do not use after the expiration date.
• The test panel should remain in the sealed pouch until use.
• The test is for single use.
• While urine is not classified by OSHA or the CDC as a biological hazard unless visibly
contaminated with blood8,9, the use of gloves is recommended to avoid unnecessary contact
with the specimen.
• The used test card and urine specimen should be discarded according to federal, state and local
regulations.
STORAGTE AND STABILITY
Store as packaged in the sealed pouch at 2-30°C (36-86°F). The test is stable through the
expiration date printed on the sealed pouch. The test device must remain in the sealed pouch until
use. DO NOT FREEZE. Do not use beyond the expiration date.
SPECIMEN COLLECTION AND PREPARATION
Urine Assay
The urine specimen must be collected in a clean and dry container. Urine collected at any time of
the day may be used. Urine specimens exhibiting visible precipitates should be allowed to settle to
obtain a clear specimen for testing.
Specimen Storage
Urine specimens may be stored at 2-8°C (36-46°F) for up to 48 hours prior to testing. For
prolonged storage, specimens may be frozen and stored below -20°C. Frozen specimens should
be thawed and mixed well before testing.
MATERIALS
Materials Provided
• Package insert • Procedure Card
• Test cup • Disposable gloves
• Color Chart Card for Adulterant Interpretation (when applicable)
Materials Required But Not Provided
· Timer
DIRECTIONS FOR USE
Allow the test cup to come to room temperature [15-30
o
C (59-86
o
F)] prior to testing.
1) Tear the foil bag open, remove test cup and disposable gloves provided for donor. Label the
device with donor information.(Fig. 1)
2) Wear disposable gloves to collect urine specimen. Open test cup lid. Urinate directly into the
Oxycodone is prescribed for the relief of moderate to high pain under pharmaceutical trade names
as OxyContin® (controlled release), OxyIR®, OxyFast®(immediate release formulations), or
Percodan® (aspirin) and Percocet® (acetaminophen) that are in combination with other
nonnarcotic analgesics. Oxycodone's behavioral effects can last up to 5 hours. The controlled-
release product, OxyContin®, has a longer duration of action (8-12 hours).
The
ECO CUP™ One Step Drug Test yields a positive result when the Oxycodone in urine
exceeds 100 ng/mL.
PHENCYCLIDINE (PCP)
Phencyclidine, also known as PCP or Angel Dust, is a hallucinogen that was first marketed as a
surgical anesthetic in the 1950's. It was removed from the market because patients receiving it
became delirious and experienced hallucinations.
Phencyclidine is used in powder, capsule, and tablet form. The powder is either snorted or smoked
after mixing it with marijuana or vegetable matter. Phencyclidine is most commonly administered
by inhalation but can be used intravenously, intra-nasally, and orally. After low doses, the user
thinks and acts swiftly and experiences mood swings from euphoria to depression. Self-injurious
behavior is one of the devastating effects of Phencyclidine.
PCP can be found in urine within 4 to 6 hours after use and will remain in urine for 7 to 14 days,
depending on factors such as metabolic rate, user's age, weight, activity, and diet.5 Phencyclidine
is excreted in the urine as an unchanged drug (4% to 19%) and conjugated metabolites (25% to
30%).
6
The
ECO CUP™ One Step Drug Test yields a positive result when the phencyclidine level in
urine exceeds 25 ng/mL. This is the suggested screening cut-off for positive specimens set by the
Substance Abuse and Mental Health Services Administration (SAMHSA, USA).
TRICYCLIC ANTIDEPRESSANTS (TCA)
TCA (Tricyclic Antidepressants) are commonly used for the treatment of depressive disorders.
TCA overdoses can result in profound central nervous system depression, cardiotoxicity and
anticholinergic effects. TCA overdose is the most common cause of death from prescription drugs.
TCAs are taken orally or sometimes by injection. TCAs are metabolized in the liver. Both TCAs
and their metabolites are excreted in urine mostly in the form of metabolites for up to ten days.
The
ECO CUP™ One Step Drug Test yields a positive result when the concentration of Tricyclic
Antidepressants in urine exceeds 1,000 ng/mL.
ADULTERANT TESTS (SPECIMEN VALIDITY TESTS) SUMMARY
The Adulterant Test Strip contains chemically treated reagent pads. Observation of the color
change on the strip compared to the color chart provides a semi-quantitative screen for oxidants,
specific gravity and pH in human urine which can help to assess the integrity of the urine
specimen.
ADULTERATION
Adulteration is the tampering of a urine specimen with the intention of altering the test results. The
use of adulterants in the urine specimen can cause false negative results by either interfering with
the test and/or destroying the drugs present in the urine. Dilution may also be used to produce
false negative drug test results. To determine certain urinary characteristics such as specific
gravity and pH, and to detect the presence of oxidants in urine are considered to be the best ways
to test for adulteration or dilution.
• Oxidants (OX): Tests for the presence of oxidizing agents such as bleach and peroxide in the
urine.
• Specific Gravity (S.G.): Tests for sample dilution. Normal levels for specific gravity will range
from 1.003 to 1.030. Specific gravity levels of less than 1.003 or higher than 1.030 may be an
indication of adulteration or specimen dilution.
• pH: tests for the presence of acidic or alkaline adulterants in urine. Normal pH levels should be
in the range of 4.0 to 9.0. Values below pH 4.0 or above pH 9.0 may indicate the sample has
been altered.
PRINCIPLE
The
ECO CUP™ One Step Drug Test is an immunoassay based on the principle of competitive
binding. Drugs which may be present in the urine specimen compete against their respective drug
conjugate for binding sites on their specific antibody.
During testing, a urine specimen migrates upward by capillary action. A drug, if present in the urine
specimen below its cut-off concentration, will not saturate the binding sites of its specific antibody.
The antibody will then react with the drug-protein conjugate and a visible colored line will show up
in the test line region of the specific drug strip. The presence of drug above the cut-off
concentration will saturate all the binding sites of the antibody. Therefore, the colored line will not
5
Adulteration Pad
Oxidants (OX)
Specific Gravity (S.G.)
pH
Reactive Indicator
0.36%
0.25%
0.06%
Buffers and Non-reactive Ingredients
99.64%
99.75%
99.94%
(Fig. 4)
(Fig. 1)
)
3
.
g
i
F
(
)
2
.
g
i
F
(
• Security seal label
5
C
T
C
T
C
T
NEGATIVE POSITIVE INVALID