K-Patents PR-23 for K-Patents Appendix User Manual
Page 5
© Copyright K-Patents 2013. All rights reserved.
5
1
This Instruction Manual Appendix covers K-Patents Pharma Refractometer PR-23-AC
when used in the production of viral vaccines. The vaccines are either produced by
inoculating viruses into specific pathogen-free eggs or in animal cell culture based process.
The allantoic fluid of these processes is harvested and purified by centrifugation and
stabilised with buffer containing sucrose. The centrifugation process typically uses density
gradient continuous flow ultracentrifuge for the purification of the virus particles. The internal
subviral core of the virus is separated and fractionated on the basis of its’ sedimentation
rate, and the buoyant sucrose density. The K-Patents Pharma Refractometer PR-23-AC is
used for accurate measurement of these sucrose densities. The measurement signal is used
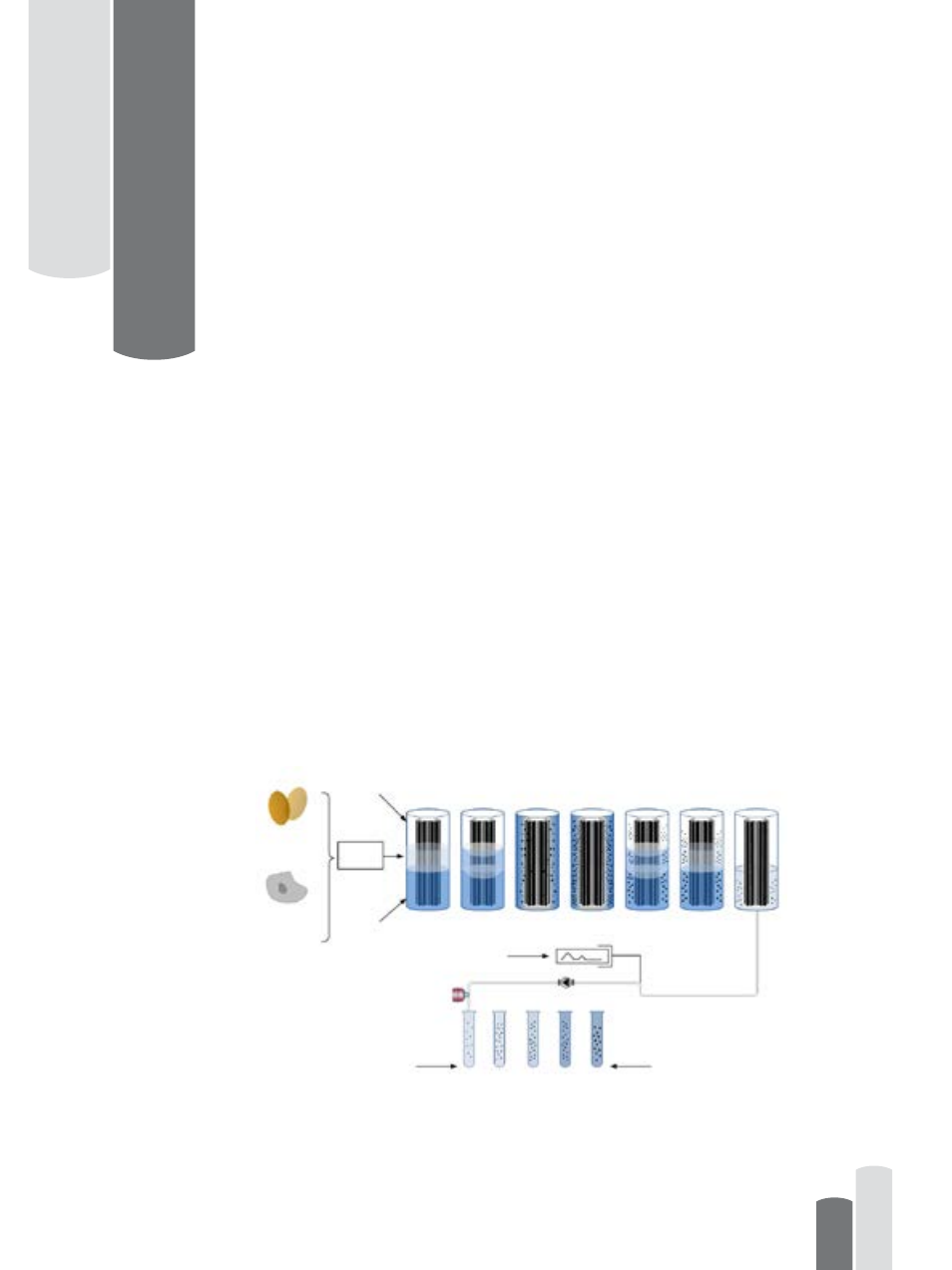
for reliable and timely determination of the product peak in the density gradient (0 to 60%
w/w sucrose), and the subsequent collection of the virus rich fraction (Figure 1.1.).
The K-Patents Pharma Refractometer PR-23-AC can be installed in the vaccines
fractionation unit for in-line process control. The output of the transmitter is a 4 to 20mA
DC output signal proportional to sucrose solution density or Brix. Process data can also be
downloaded to a computer via an Ethernet cable.
High Density
Solution
• Inoculation egg
with virus
• Incubation
• Inoculation
preparation
• Cell expansion
• Virus propagation
Low Density
Solution
Low Density
High Density
Filling
Fractionation and separation
Chart Recorder
Rotor unloading
Sucrose gradient purification by zonal centrifugation
Inactivation
of virus
Figure 1.1 Ultracentrifugation density gradient purification process steps.
K-Patents Products for Vaccine
Production and Sucrose Gradient
Ultracentrifugation