Hoefer SE1200 User Manual
Page 17
4
Pour 5 – 10 ml of distilled water around the edges of
and onto the gel. Cover the gel with a fully hydrated
sheet of either cellophane or mylar:
Cellophane. Use for fluorography, thick gels, or to
preserve a gel. Fully hydrate a sheet of porous cello-
phane in distilled water and place it over the gel.
(Cellophane does not quench the signal of fluor-
treated gels.)
Mylar. Use for contact autoradiography for gels
≤1 mm. Fully hydrate a sheet of Mylar in distilled
water and place it over the gel. Once the gel is dried,
the Mylar peels off. Place the uncovered side against
the X-ray film.
5
Position the outer frame — with the Hoefer label facing
up and the locking knobs facing down — over the
cellophane sheets and around the inner frame. Press
down to engage the two frames. Applying pressure
to opposite corners of the frame usually causes the
frames to slip easily into place.
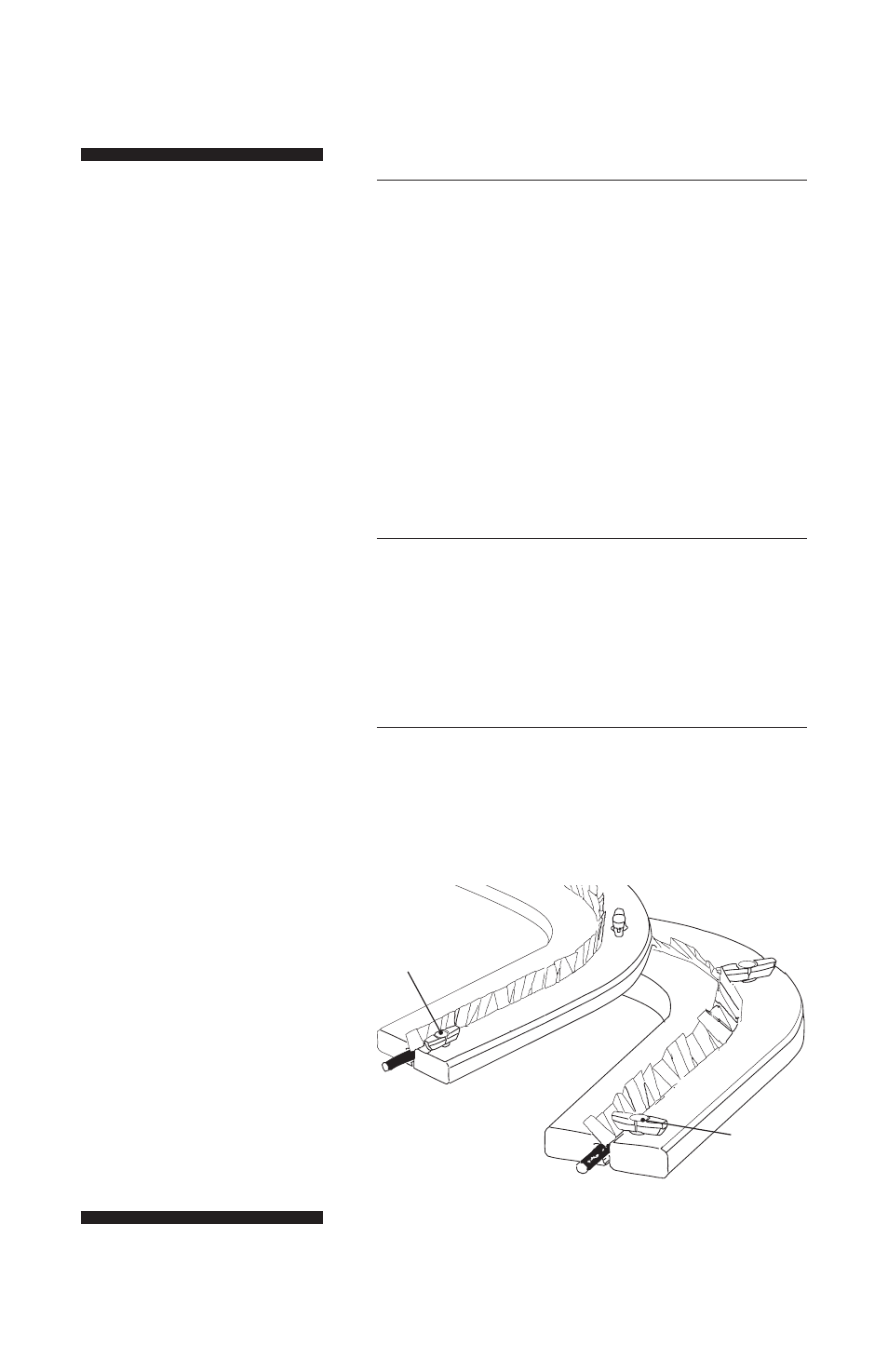
6
Turn the frame assembly over. Turn each knob 1/4
turn so that the long edge is perpendicular to both
frames. (See Fig 3.) This “locks” the two frames
together, preventing slippage as the gel shrinks
during drying.
•
p8
Important! Remove all bubbles
trapped under the top sheet by
displacing them with distilled
water and pushing them to the
edge of the gel with your gloved
finger. Work from the center of
a single gel or, if several gels
are on the platform, work from
the center of the frame toward
the edge.
Fig 3. Lock the frame flip the
assembly over and turn the knobs
perpendicular to the frames.
Open
Closed