2 carbcalcii introduction – Super Systems CarbCALC II User Manual
Page 5
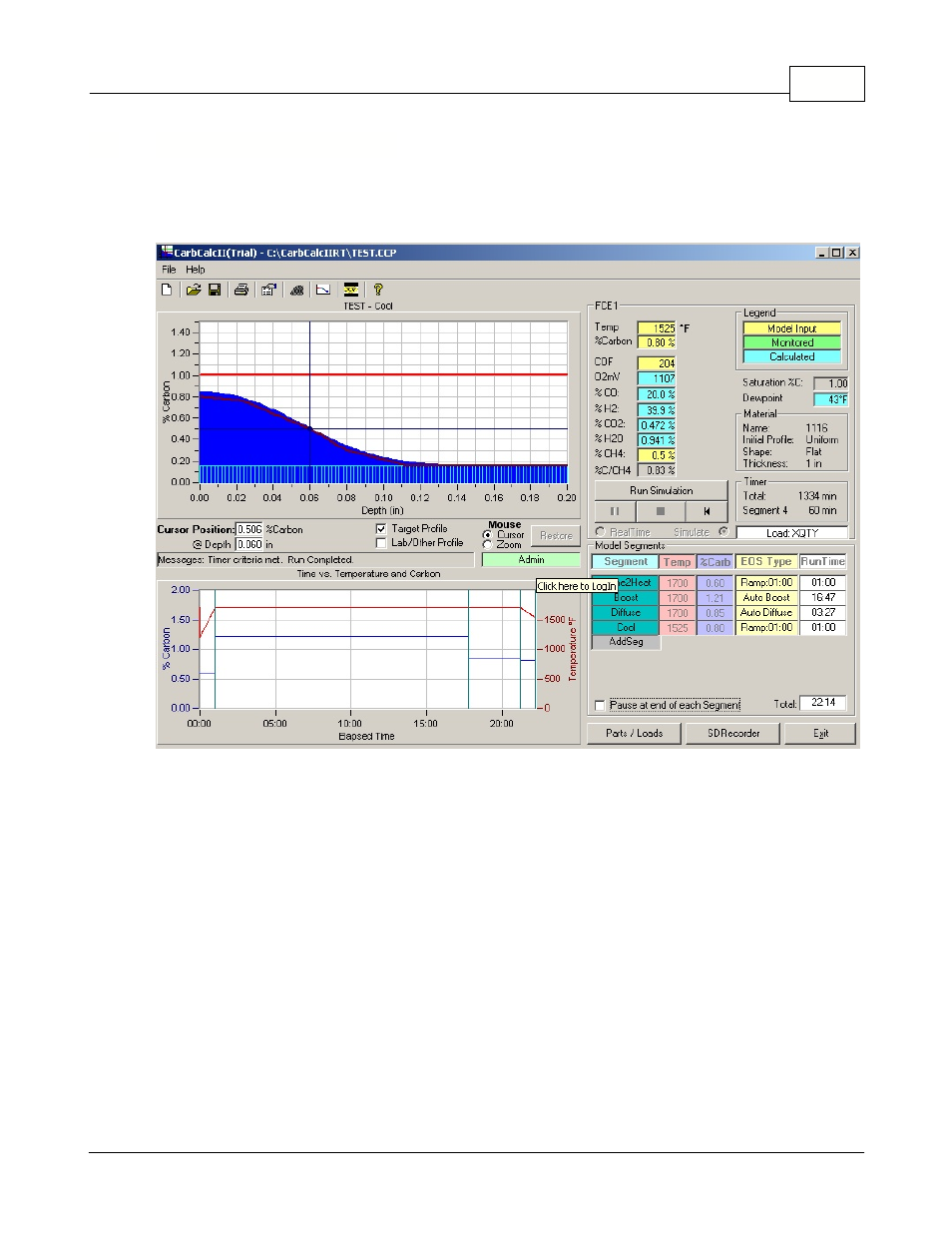
Introduction
CarbCalc II
4
© 2005,2006,2007 Super Systems Inc.
1.2
CarbCalcII Introduction
CarbCalcII is a Carbon Diffusion Model for use with Batch Furnace Gas Carburizing.
Gas Carburizing is a case-hardening process in which carbon is dissolved in the surface layers of a
low-carbon steel part at a temperature sufficient to render the steel austenitic, followed by quenching
and tempering to form a martensitic microstructure. The resulting gradient in carbon content below the
surface of the part causes a gradient in hardness, producing a strong, wear-resistant surface layer on
a material, usually low-carbon steel, which is readily fabricated into parts. In gas carburizing the source
of carbon is a carbon-rich furnace atmosphere produced either from gaseous hydrocarbons, for
example, methane (CH4), propane (C3H3), and butane (C4H10), or from vaporized hydrocarbon
liquids.
Carbon Sources
Low-carbon steel parts exposed to carbon-rich atmospheres will carburize at temperatures of 850°C
(1560°F) and above. If the carbon source is so rich that the solubility limit of carbon in austenite is
reached at the surface of the steel some carbides may form at the surface. At these "above saturation"
atmospheres soot will deposit on surfaces within the furnace, including the parts. The goal of modern
gas carburizing practice is to control the carbon content of furnace atmospheres such that: The final
carbon concentration at the surface of the parts is below the solubility limit in austenite and Sooting of
the furnace atmosphere is minimized. Endothermic gas (Endogas) is a blend of carbon monoxide,
hydrogen, and nitrogen (with smaller amounts of carbon dioxide water vapor, and methane) produced
by reacting a hydrocarbon gas such as natural, gas (primarily methane), propane or butane with air. A